This is the continuation of m pharm pharmacology jobs part 2
Click here to view the part 2
6. Online publisher companies:
These types of jobs also available for every filed of pharmacy. Pharmacology graduates work for the clinical, medical and life sciences related journals. After receiving a few manuscripts per day, you have to correct, edit, accept or reject the manuscript for publishing. It is a systematic process, if you are doing you may be promoted to curator to reviewer(the next level of designation). This is a desktop job.
Other conference conducting companies are also available. Usually the same company work as an online publisher along with conducting the conferences. These two are interlinked. You are aware of conferences like IPC(Indian Pharmaceutical Congress), World Pharma Congress etc. (Probably you are already attended some conference in your academics). M Pharmacy graduates are recruited to co-ordinate and manage the conferences, summits. Work is inviting the related scientists, professors and scientific personnel’s through mails and calls. If you worked well, finishing the targets effectively and went to next level like conference co-ordinator(Team Leader), you may go to foreign countries (where your conference is scheduled like newzeland, Australia etc) a few days for coordinating the conference. Communication, management skills are important for this type of job. The working hours will be a little change depend upon which country conference you are working for. Here also there is a chance of bulk recruitment drive if they need work force. If you need any further guidance ask your questions in the comment box or contact us through mail.
7) CDM:
M pharm pharmacology jobs are available in CDM. CDM is known as Clinical Data Management. It deals with the clinical data by applying various computer based applications. It involves collecting, processing , cleaning and management of subject or clinical trial data according to the project specific guidelines. It usually contains 3 phases like startup, conduct and close out phase. It starts from crf@ and database design to database lock.
Coming to M pharm pharmacology jobs, Jr. Clinical Data Associate (Jr. CDA) designated vacancies are available for freshers. You can learn the CDM course or you can directly face the interview without doing any additional course after M pharm. You should have the good knowledge on clinical trials and its phases.Every sponsor company will not have this CDM department on their own or they will outsource their work to Pharmaceutical service providing IT companies. If the sponsor have more stringent regulatory products in the market or they are focusing on new drug applications they have their own teams. Even other M pharm graduates can also try for this jobs, but pharmacology graduates are preferred.
@ CRF is known as Clinical Report Form, It is essential for recording the clinical trial data. Paper CRF(Manual) or eCRF(electronic)s are types of CRFs.
8) Pharmacovigilance:
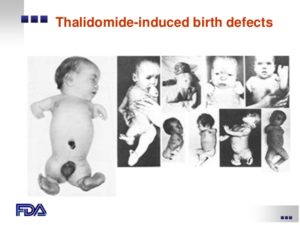
It deals with the adverse reactions and helps to improve the patient care and public safety in relation to the use of drugs. This concept(Drug safety and adverse drug reaction reporting) was more concentrated from thalidomide disaster@@ onwards(after 1961). Two types of ADR reporting is done. They are spontaneous reporting and mandatory reporting.
Coming to the m pharm pharmacology jobs, Drug Safety Associate designated vacancies are available. Job profile includes extracting and submitting the ICSRs(Individual Case Safety Reports), Literature surveillance of ADRs, Signal detection(is known as reporting the casual association of drug with adverse event, which is not clearly reported previously), preparing the PSURs(Periodic Safety Update Reports). These are all regulatory requirements for detecting, assessing and preventing the adverse reactions of drugs to improve patient safety. Different types of software tools are used in pharmacovigilance. E.g Vigi flow, ARISg, Argus, Vigibase etc. These tools are preferred based the sponsor’s marketed product approved regulatory authority. Like ARISg is mostly used by drug manufacturers in Europe, Argus is used by drug manufacturer in USA and VigiFlow is used by national pharmacovigilance centres of the WHO Programme for International Drug Monitoring. You can learn the pharmacovigilance course otherwise you can directly try for the interview. Although other m pharmacy graduates are eligible for this job, pharmacology graduates are preferred for this job.
@@Thalidomide is a teratogen, it is used to prevent morning sickness during pregnancy in nearly 40 countries. It caused birth defects (phocomelia) in thousands of babies(more 10,000 birth defects in Germany itself).
Conclusion:
These are the possible job opportunities after m pharm pharmacology. Choosing a correct job is depends upon your interest and your priorities only. Every type of above mentioned fields have their own advantages and disadvantages. It may include form salary, career growth in future, work shift timings etc. Please try to get a job which fits you well. Doing your interested job gives you satisfaction. I hope this information will help to comparing and selecting the suitable job for our pharmacology graduates.
I have seen some practical cases in my life, some people will join in one of the above mentioned filed. Getting 1or 2 years experience, they may shift to another filed which they are actually interested. It will not only waste the time but also their career, because the working experience will not be considered for the second field, again it will start from zero experience and growth. It will happens because of lack of knowledge related to job opportunities. So carefully select your first job and build your career successfully.
Please share your opinions below