Clinical trials all about conducting clinical research about new drug or device in humans. Every objective of the each phase of clinical trial is different from each other. Before getting into the clinical research in the humans, a lot of laboratory research already completed from the previous time that is preclinical research involving animals.
It is a complete step by step process like a constructing a building by using bricks and cement. Here bricks and cement are scientifically generated proofs. All procedures conducted according to the guidelines and accepted methods with prior approvals. Produces scientifically accepted proof to proceed for further research.
Related: Download free pharmacology books
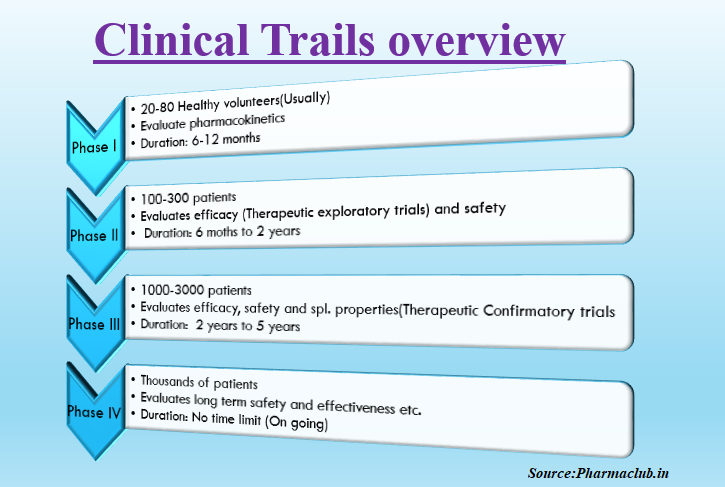
Clinical trials are divided into four phases. Four phases are as follows.
Phase I :
This is the first phase of human clinical trials. Some times Phase 0 (micro dosing) studies are also considered as first phase in human trails. Phase 0 trials not mandatory for all kinds of studies. But it helps to take the decision to proceed for further trials or not.
Usually phase I trials conducted in healthy population except for few diseases like HIV and cancer. It assess the new drug safety. 20-100 healthy volunteers tested for few months in this phase. Drug Pharmacokinetics tested here like how the drug affecting the body by knowing absorption, distribution, metabolism and excretion. Along with pharmacokinetics, it finds what are the side effects of the drug if dose level increased. Usually Seventy percentage of molecules can successfully complete this phase.
Phase II:
This is the second phase of human clinical trials. It studies the drug efficacy. From this phase testing will be done on patients. Usually hundreds of patients tested for few months to two years in this phase. Number of patients used in this phase depends upon required statistical sample size calculation. Experimental drug efficacy compared with either standard drug or placebo. Relative experimental drug safety and efficacy compared by using double blind study designs. After entering drugs into the phase II, 30-40% molecules can successfully complete this phase.
Phase III:
It studies the effectiveness of a drug. It is a very large scale testing involves several thousands of patients tested for up to several years. Thorough information of a drug observed here including adverse effects from various study designs and testing procedures. Once this phase successfully completed, research company can request for the regulatory authorities for drug marketing approval After entering the drug into phase III, 50-60% molecules can successfully complete this phase.
Phase IV:
This phase starts after drug approval for sale. This phase includes post marketing surveillance trails. Different types of findings observed about a drug in this phase like rare adverse reactions, drug interactions, long term safety, effectiveness and effect on patients quality of life, cost effectiveness with other therapies. Depend upon these findings, the regulatory authorities may recall or restrict the use or continues the drug in the market.
For example: Vioxx (Rofecoxib) is a nonsteroidal anti-inflammatory drug manufactured from Merck limited for pain relief. It was in the market for about 5.3 years. Due to findings in Phase IV like increased risk for heart attack and stroke, It was recalled from the market by FDA in 2004.
Zelnorm (Tegaserod maleate), a Novartis product indicated for irritable bowel syndrome with constipation in women younger than 55. Zolnorm also in the market for about 4.6 years in the market. Recalled by FDA in 2007 with a similar findings in Phase IV trials.
Related: Opportunities for pharmacology students