Pharmaceutical companies
Pharmaceutical companies play a vital role in the healthcare sector. It discovers, develops and manufacture the formulations for a wide variety of diseases. It include from a simple diseases/ conditions to a complex or chronic conditions/diseases. These drugs helps to prevent, control or treat these diseases/conditions. Development of a drug involves a wide variety of processes. Depend upon their plants activity, the overall pharmaceutical companies can contain following types of facilities/divisions as shown below
1) Manufacturing units
2) Research and Development units
3) Marketing units
Related: Pharmaceutical industries list
Remember depend upon the company’s investment capacity, equipment setup and their interest towards the products or diseases, some companies contains all the above-mentioned facilities. These are called as Brand developers. These are all usually large and Multinational companies(MNCs). Some other companies may only dedicated to marketing or manufacturing or research and development services or combination of services.
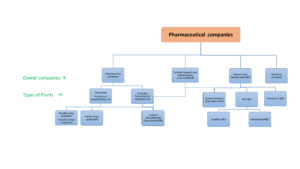
1) Pharmaceutical manufacturing units/plants:
Pharmaceutical manufacturing unit/plant is a broad term; It will not indicate any specific facility. Several sub types are available depend upon the type of product preparation. This can be divided as three types
A) Formulation manufacturing plant
B) API manufacturing plant
C) Contract Manufacturing Organisations(CMO)
A) Formulation manufacturing plant:
This plant can also called as a Formulation production plant. Formulation/ Finished Dosage Form (FDF) is a final product from the manufacturing facility, which is ready for marketing. After receiving the approval, these facilities will start production of the formulation. These formulations can also be divided into two types from the regulatory point of view. It includes
Generic formulation
Branded drug formulation.
There will not be a specific facility dedicated to produce either generic or branded drug formulations. Branded and generic drugs can be produced in one plant. Usually smaller companies will produce generic formulations and larger companies will produce branded formulations along with generic formulations. Before producing either of these formulations, the plant and the formulation should have a respected regulatory authority approval.
B) API manufacturing plant:
This can be also called as API Production plant or Bulk drug production plant. API is known Active Pharmaceutical Ingredient. To prepare any dosage formulation API is the very important ingredient. There is no formulation available without API (A Formulation without API is known as Placebo, it does not have any therapeutic action).
Pharmaceutical intermediates can also be produced in bulk production plants. Intermediates are nothing but building blocks for the synthesis of Active Pharmaceutical Ingredient. These API/Intermediates sold to pharmaceutical companies for production of finished formulation. Depend upon their followed guidelines and accreditations from various regulatory bodies, they can export their materials to the different countries.
E.g: Arch pharmalabs
C) Contract Manufacturing Organisations(CMO)
Contract Manufacturing Organisation is a segment of CRAMS (Contract Research And Manufacturing Services.)
CRAMS:
Pharmaceutical or biotechnology industry uses the services by outsourcing to the CRAMS. Larger pharmaceutical companies outsource some of their work to CRAMS to avoid the increasing cost, time and low productivity. India offers the cost advantages and with over mature manufacturing hubs.
Contact Manufacturing Organisation offers the manufacturing services to the various pharmaceutical or biotechnology companies. CMO manufactures Active Pharmaceutical Ingredients(API) or Finished Dosage Forms. It prepares various dosage forms from small quantities to larger quantities. It depends upon the sponsor(client) requirement. The requirement may be small volume for preclinical or clinical purposes (PhaseI/IIor III) to larger volumes for commercial need. According their need CMOs will be chosen. To get more clear idea click here
e.g Aether Industries
2) Research and Development(R&D) plant:
It is the heart and soul for any product development. Research will start from getting thorough knowledge of their interested molecule/formulation. Several trial batches conduced for developing a promising formulation or API or molecule. This promising formulation is executed in manufacturing plant after successful completion of stability studies. R&D plants can be divided into following types depend upon their activities.
A) NCE R and D:
New Chemical Entity is simply defined as New active moiety/ingredient which is not yet approved by any regulatory authority till now. Preparing a new chemical entity involves an expensive, lengthy and complicated procedure. It involves Drug discovery, pre-clinical and clinical development. These processes need to be completed successfully for marketing a new chemical entity formulation. It will take time at least a minimum of one decade.
Synthetic R&D:
We can call it as Process R&D(PRD). Synthetic R&D deals with researching and developing of an Active Pharmaceutical Ingredient(API).
Bioanalytical R&D:
This also involved in drug discovery. Bioanalytical R&D deals with the method development and analysis of different compounds which are using in the preclinical and clinical research.
B) Formulation R&D:
This can be called as FR&D or FD(Formulation Development). Research focused on developing the new formulations, it may be a branded or generic formulation depend upon the company’s interest and investment. In case of generics, one formulation prepared as a branded formulation after expiring its market exclusivity from a parent company. Apartment from branded formulations, branded generics also developed. There is no specific R&D is dedicated to develop only Generics or only branded formulations. Both formulations can be developed on one R&D depend upon the company interest.
C) Contract Research Organisation(CRO):
Like a CMO, CRO is a segment of CRAMS(Contract Research and Manufacturing Services). CRO provides the research and clinical trial services to sponsor companies or institutions It may include pharmaceutical companies, biotechnology companies or institutions. Most of their contracts come from pharmaceutical and biotechnology companies. Remaining contracts may come from medical device companies, institutions or research foundations. If sponsor outsourced their work to CRO, sponsor is the responsible party for data integrity. To get more clear idea click here
e.g: Quintile, Parexel, Vimta labs etc
3) Pharmaceutical marketing companies:
A few companies especially dedicated to marketing services only known as “Trade companies”. It takes manufactured products from the pharma companies and sell in the market. Solely pharma based marketing companies have established presence in the market. It helps a lot to push the product into the market effectively.
e.g Daksh Pharmaceuticals.
Some companies sell their manufactured products on their own. Even if they have own marketing team, they may give their some of their manufactured products to some other trading companies depend upon their presence in the market. If you observe the back of the formulation package strip, two things observed here; i.e manufactured by and other one marketed by/distributed by. If the formulation is from abroad you can observe imported and marketed by.
Note: Here we haven’t discussed anything about Biotechnology companies.
Please share your opinion below..